Chaperone Protein Htpg
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
VAL-208
GLU-209
4.4
4.0
-1.4
2.4
38.2
39.6
-4.6
GLU-209
ILE-210
3.0
2.8
-3.0
4.8
31.6
31.4
-11.4
ILE-210
GLU-211
3.1
2.7
0.3
19.1
131.5
128.7
-132.3
THR-220
VAL-221
8.7
6.3
48.2
-23.8
13.0
59.4
37.2
THR-220
VAL-221
8.7
6.3
48.2
-23.8
13.0
59.4
37.2
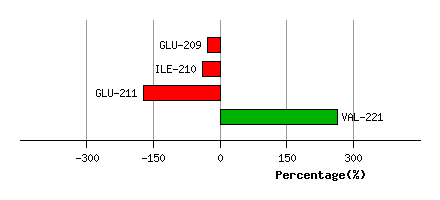
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
THR-220
VAL-221
8.7
6.3
48.2
-23.8
13.0
59.4
37.2
THR-220
VAL-221
8.7
6.3
48.2
-23.8
13.0
59.4
37.2
VAL-221
ILE-222
9.1
8.5
33.0
10.8
63.0
43.8
415.6
ILE-222
SER-223
6.5
8.1
-66.0
14.3
152.1
142.9
-519.1
SER-223
TRP-224
7.2
7.8
-17.0
-5.4
120.3
120.4
-166.9
TRP-224
GLU-225
5.0
5.6
6.9
-23.9
71.8
73.4
-67.4
GLU-225
LYS-226
6.7
6.8
-14.5
13.5
138.5
125.5
92.5
LYS-226
ILE-227
7.1
7.0
-14.3
3.7
78.8
73.3
15.2
ILE-227
ASN-228
9.0
8.5
-38.0
66.2
114.5
127.3
166.7
ASN-228
LYS-229
11.5
10.7
-9.2
-0.1
94.8
91.8
-47.7
LYS-229
ALA-230
13.5
13.2
-6.2
0.9
155.3
155.4
-57.1

Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees